Reducing hospitalisation in early-phase schizophrenia – Results of the PRELAPSE study[1]
In the first of two webinars, John Kane, Professor and Chairman of Psychiatry at The Donald and Barbara Zucker School of Medicine, New York, USA, provided a rationale for the use of long-acting injectable (LAI) antipsychotics in early-phase schizophrenia. He then introduced PRELAPSE1, a large-scale, multicentre LAI study of patients with early-phase schizophrenia, which, by challenging perceptions that LAIs are a treatment of ‘last resort’ with a low patient acceptance rate, should have implications for future clinical practice.

Professor John Kane
Professor John Kane
Professor Kane said it is widely recognised that antipsychotic medication is highly efficacious, citing a meta-analysis2 that compared various medications with placebo for the prevention of relapse and found the number needed to treat (NNT) was 3. He said the challenge was how to ensure patients continue taking their medication regularly when this is a struggle for many people living with chronic illness.3,4 Professor Kane said that relapse rates are high, even after one episode, referencing a study that showed 82% of patients had a relapse within 5 years.5 Patients who discontinued their medication were almost 5 times more likely to relapse compared with those who continued treatment.5
The importance of preventing relapse
Patients are at risk of experiencing disease progression after each relapse,6 and their response rates to medication can be significantly reduced.7 Professor Kane said other possible consequences of first and second relapses in early-phase schizophrenia included increased use of healthcare resources, long-term symptoms and social disability, increased risk of self-harm, and increased burden on care givers.8 He believes not continuing antipsychotic medication after a first episode is risky, and noted that continued treatment increases the chance of survival compared with stopping medication.9

The rationale for using LAIs
It has been suggested that LAIs may be more efficacious than their oral counterparts: a Finnish cohort study10 of selected antipsychotics (that did not assess Abilify Maintena) found that within 60 days of leaving hospital, less than half (45.7%) the patients who had experienced a first episode were adhering to their antipsychotic medication. In that study’s pairwise comparisons between four LAIs and their equivalent oral formulations, no statistically significant differences in rehospitalisation risk were observed between pairs. However, in its pooled analysis, the LAIs were associated with a statistically significant lower risk of rehospitalisation (p=0.007).10

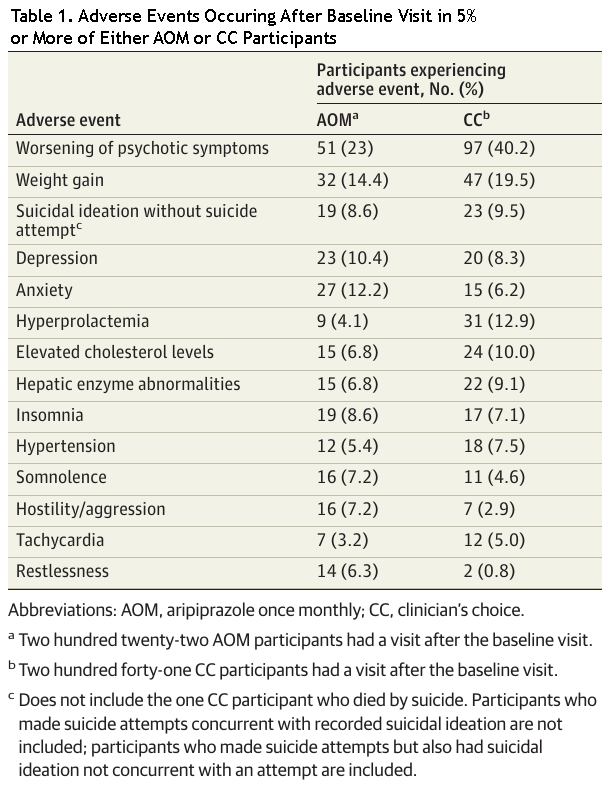
Prescribers should consult the approved Australian Product Information documents for full safety and tolerability details.12
Prescribers should consult the approved Australian Product Information documents for full safety and tolerability details.12
The PRELAPSE study
The Prevention of Relapse in Schizophrenia (PRELAPSE) study was an investigator-initiated, multicentre, cluster-randomised clinical trial with a 2-year follow-up.1
The objective for PRELAPSE was to understand the acceptability of treatment with LAIs in patients with early-phase or first episode schizophrenia and to determine if encouraging the use of an LAI antipsychotic delays the time to first psychiatric hospitalisation compared with clinician’s choice of treatment.1,11
Professor Kane said that Abilify Maintena® (aripiprazole) once monthly12 was chosen as the study medication as studies have shown it is a well-tolerated medication.11 He believes tolerability is very important in early-phase illness; patients are more sensitive to adverse drug effects and so choosing a treatment they can tolerate may contribute to therapeutic compliance.13 Patients in the study’s comparator arm received their clinicians’ choice of therapy.1
A total of 489 eligible patients aged between 18–35 years with confirmed schizophrenia and less than 5 years of antipsychotic use received either LAI treatment with Abilify Maintena® or clinician’s choice, depending on the site at which they were treated.1 It should be noted that clinician’s choice could include other commonly prescribed antipsychotic medication, including LAIs. The primary outcome measure was time to first psychiatric hospitalisation.1
Professor Kane said a critical component of the study was that the treatment centres chosen to treat patients with Abilify Maintena® were provided with detailed training. This included explaining the rationale for LAI use, the role of non-adherence in relapse and hospitalisation, shared-decision making principles and role playing a variety of clinician–patient scenarios.11 In this study, 91.0% of patients agreed to receive at least one injection.1
At the conclusion of the study, it was found that the mean survival time until first hospitalization was extended for first episode and early-phase patients in the Abilify Maintena® group, compared with clinician’s choice (P value not reported).1 The NNT was 6.67 for the prevention of one hospitalisation, and a 44% reduction in the incidence rate of first hospitalisation compared with the clinician's choice group was observed (P=0.02).1
Key Points
- Relapse rates are high in first-episode or early schizophrenia, even after one episode5
- Relapses have serious consequences, particularly in the early phase of illness1
- Patients who discontinued their medication were almost 5 times more likely to relapse compared with those who continued treatment.5
- In a Finnish study, use of selected LAIs (Abilify Maintena® was not assessed) in early-phase schizophrenia was associated with beneficial clinical outcomes, including reduced risk of rehospitalisation compared with oral formulations, and mortality compared with no antipsychotic medication use10
- In the PRELAPSE study, patients at Abilify Maintena® sites showed a 44% reduction in the incidence rate of first hospitalisation and an NNT of 6.67 for the prevention of hospitalisation over a two-year period compared with clinician’s choice (P=0.02)1


